The recalled dietary supplements contain iron, which must be in child-resistant packaging, as required by the Poison Prevention Packaging Act. The iron-containing dietary supplement packages violate the federal standard for child-resistant packaging because the bottles and blister packs are not child-resistant, posing a risk of deadly poisoning, if the contents are swallowed by young children.
About 60,000
iHerb toll-free at 888-430-4770 at any time, email at ProductRecall@iherb.com, or online at https://www.iherb.com/help/contact or at https://information.iherb.com/hc/en-us/articles/37894456653972-iHerb-Recalls-California-Gold-Nutrition-Iron-Supplements-Due-to-Risk-of-Child-Poisoning for more information.
Recall Details
This recall involves three types of California Gold Nutrition dietary supplements: Daily Prenatal Multi, Ultamins Women’s Multivitamin, and Ultamins Women’s 50+ Multivitamin.
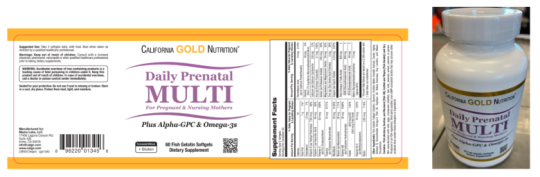
The California Gold Nutrition Daily Prenatal Multivitamin bottles are white with a white lid and a gold border label. The bottle contains 60 fish gelatin softgel dietary supplements.
The California Gold Nutrition Women’s Multivitamin and Women’s 50+ Multivitamin both have dark purple packaging with a gold border label. Both products contain 60 capsules in foil within the packaging. The following batch numbers and expiration dates for the products are printed on the back of the package near the bottom of package/sleeve.
| Products | Batch Codes | Expiration Dates |
|---|---|---|
| Daily Prenatal Multi | 2307050A, 2404096A, 2411100A | 08/2025, 05/2026, 11/2026 |
| Ultamins Women’s Multivitamin | V0532, V0533 | 11/2026, 07/2026 |
| Ultamins Women’s 50+ Multivitamin | V0534, V0536 | 07/2026, 11/2026 |
Consumers should immediately secure the recalled supplement bottles out of sight and reach of children and contact iHerb for a refund and information on how to safely discard the packaging and product. To receive a refund, consumers should email ProductRecall@iherb.com with the subject line: “Iron Supplement Refund” and provide the following information:
- Name
- Product(s) name & quantity ordered
- Evidence of destruction: initial and date each product package and include a photo with your initials and the date visible
- Optional: Provide the order number associated with the purchase(s), which can be found by logging into consumer’s account and viewing the “Order History”.
iHerb is contacting all known purchasers directly.
None reported
Note: Individual Commissioners may have statements related to this topic. Please visit www.cpsc.gov/commissioners to search for statements related to this or other topics.
If you are experiencing issues with a recall remedy or believe a company is being non-responsive to your remedy request, please use this form and explain the situation to CPSC.
The U.S. Consumer Product Safety Commission (CPSC) is charged with protecting the public from unreasonable risk of injury associated with the use of thousands of types of consumer products. Deaths, injuries, and property damage from consumer product-related incidents cost the nation more than $1 trillion annually. Since the CPSC was established more than 50 years ago, it has worked to ensure the safety of consumer products, which has contributed to a decline in injuries associated with these products.
Federal law prohibits any person from selling products subject to a Commission ordered recall or a voluntary recall undertaken in consultation with the CPSC.
For lifesaving information:
- Visit CPSC.gov.
- Sign up to receive our email alerts.
- Follow us on Facebook, Instagram, X, BlueSky, Threads, LinkedIn and Truth Social.
- Report a dangerous product or product-related injury on www.SaferProducts.gov.
- Call CPSC’s Hotline at 800-638-2772 (TTY 800-638-8270).
- Contact a media specialist.