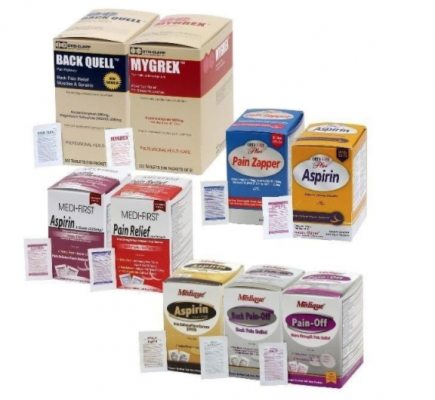
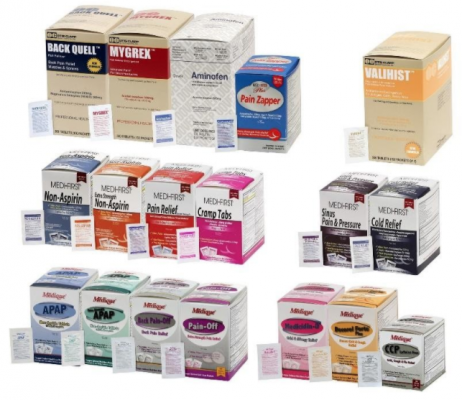
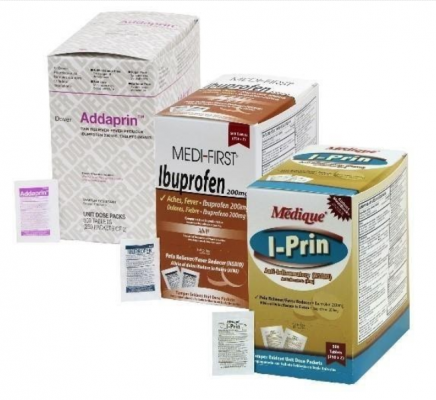
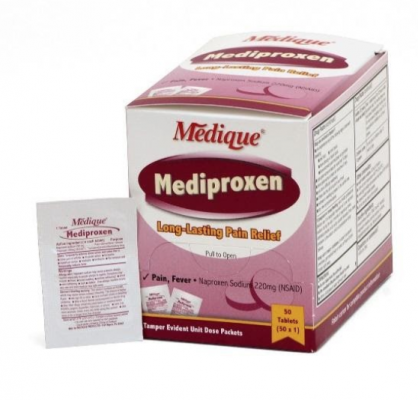
The over-the-counter products contain regulated substances which must be in child resistant packaging when being used in the household as required by the Poison Prevention Packaging Act (PPPA). The packaging of the products is not child resistant, posing a risk of poisoning if the contents are swallowed by young children.
About 143,300
Medique at 800-634-7680 from 8 a.m. to 7 p.m. ET, Monday through Friday, or online at www.mediqueproducts.com and click on "Recall Notice" at the bottom of the page for more information including registration access.
Recall Details
The recall involves 31 different over-the-counter drugs purchased on or after June 1, 2018 that are unexpired from the following brands: Medi-First, Medi-First Plus, Medique, Dover, Otis Clapp, and Ecolab. They include aspirin-containing products, acetaminophen-containing products, ibuprofen-containing products, lidocaine-containing products, diphenhydramine, loperamide, and naproxen products. The expiration date for tablets and creams can be found on either the top or side panels of the container carton in the format [YEAR/MO]. For products in spray bottles, the expiration date in the same format is located on the front of the bottle. The expiration date is found on the bottom for the spray cans. The 31 different recalled products are listed in the table below:
|
Product |
Drug |
Package Type |
# of Packets |
|
Medi-First Non-Aspirin Acetaminophen |
acetaminophen (325 mg) |
2 tablets packet |
50 250 |
|
Medi-First Extra Strength Non-Aspirin Acetaminophen |
acetaminophen (500 mg) |
2 tablets packet |
50 125 250 |
|
Medi-First Sinus Pain & Pressure |
acetaminophen (500 mg) |
2 tablets packet |
50 125 250 |
|
Medique APAP |
acetaminophen (325 mg) |
2 tablets packet |
250 |
|
Medique Extra Strength APAP |
acetaminophen (500 mg) |
2 tablets packet |
50 125 250 |
|
Medique Back Pain-Off |
acetaminophen (250 mg) |
2 tablets packet |
50 100 250 |
|
Medique CCP Caffeine Fee |
acetaminophen (325 mg) |
2 tablets packet |
50 250 |
|
Medi-First Cold Relief |
acetaminophen (325 mg) |
2 tablets packet |
50 125 250 |
|
Medique Cramp Tabs |
acetaminophen (325 mg) |
2 tablets packet |
50 125 250 |
|
Medique Decorel Forte Plus |
acetaminophen (325 mg) |
2 tablets packet |
50 250 |
|
Medique Medicidin-D |
acetaminophen (325 mg) |
2 tablets packet |
50 100 250 |
|
Dover Aminofen |
acetaminophen (325 mg) |
2 tablets packet |
250 |
|
Otis Clapp Back Quell |
acetaminophen (200 mg) |
2 tablets packet |
150 |
|
Otis Clapp Mygrex |
acetaminophen (500 mg) |
2 tablets packet |
150 |
|
Otis Clapp Valihist |
acetaminophen (325 mg) |
2 tablets packet |
150 |
|
Medi-First Pain Relief Extra Strength |
acetaminophen (110 mg) |
2 tablets packet |
50 100 250 |
|
Medi-First Plus Pain Zappers |
acetaminophen (250 mg) |
2 tablets packet |
50 125 |
|
Medique Pain-Off |
acetaminophen (250 mg) |
2 tablets packet |
50 100 250 |
|
Medi-First Aspirin |
aspirin (325 mg) |
2 tablets packet |
50 125 250 |
|
Medi-First Plus Aspirin |
aspirin (325 mg) |
2 tablets packet |
50 125 |
|
Medique Aspirin |
aspirin (325 mg) |
2 tablets packet |
12 100 250 |
|
Medique Diphen |
diphenhydramine (25 mg) |
1 tablet packet |
24 200 |
|
Medi-First Ibuprofen |
ibuprofen (200 mg) |
2 tablets packet |
4 50 125 250 |
|
Medique I-Prin |
ibuprofen (200 mg) |
2 tablets packet |
3 100 250 |
|
Dover Addaprin |
ibuprofen (200 mg) |
2 tablets packet |
250 |
|
Medi-First Burn Cream with Lidocaine |
lidocaine (0.9 grams) |
packets |
25 |
|
Medi-First Burn Spray |
lidocaine HCl (2%) |
2 oz bottle |
-- |
|
Medi-First Blood Clotting Spray |
lidocaine (4%) |
3 oz bottle |
-- |
|
Ecolab Burn Cream |
lidocaine (0.9 grams) |
packets |
25 |
|
Medique Diamode |
loperamide HCl (2 mg) |
1 tablet packet |
6 50 100 |
|
Medique Mediproxen |
naproxen sodium (220 mg) |
1 tablet packet |
50 100 |
Consumers should immediately store the recalled products in a safe location out of reach of children and contact Medique for information on how to dispose of the product and receive a full refund. All known purchasers are being notified directly.
None reported.
Note: Individual Commissioners may have statements related to this topic. Please visit www.cpsc.gov/commissioners to search for statements related to this or other topics.
If you are experiencing issues with a recall remedy or believe a company is being non-responsive to your remedy request, please use this form and explain the situation to CPSC.
The U.S. Consumer Product Safety Commission (CPSC) is charged with protecting the public from unreasonable risk of injury associated with the use of thousands of types of consumer products. Deaths, injuries, and property damage from consumer product-related incidents cost the nation more than $1 trillion annually. Since the CPSC was established more than 50 years ago, it has worked to ensure the safety of consumer products, which has contributed to a decline in injuries associated with these products.
Federal law prohibits any person from selling products subject to a Commission ordered recall or a voluntary recall undertaken in consultation with the CPSC.
For lifesaving information:
- Visit CPSC.gov.
- Sign up to receive our email alerts.
- Follow us on Facebook, Instagram, X, BlueSky, Threads, LinkedIn and Truth Social.
- Report a dangerous product or product-related injury on www.SaferProducts.gov.
- Call CPSC’s Hotline at 800-638-2772 (TTY 800-638-8270).
- Contact a media specialist.