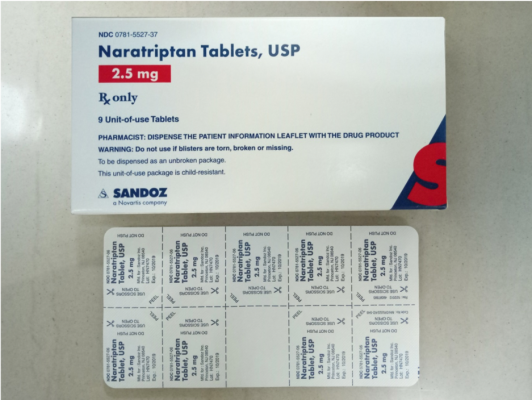
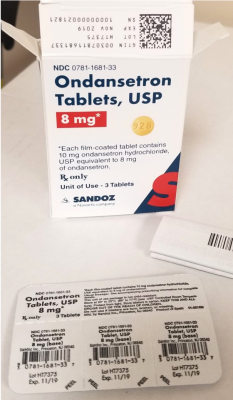
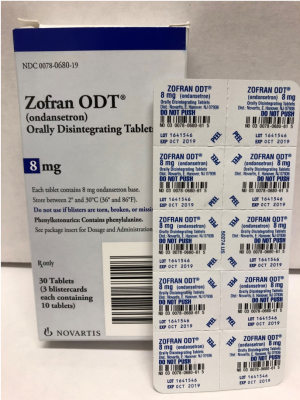
The prescription drug packaging is not child resistant as required by the Poison Prevention Packaging Act, posing a poisoning risk if swallowed by children.
About 470,000
Sandoz and Novartis toll-free at 888-669-6682 from Monday to Friday, 8:30 a.m. to 5 p.m. ET or online at www.us.sandoz.com and click on “Patients and Customers” then “Product Safety Notices,” or at www.pharma.us.novartis.com and click on banner “Novartis recalls select product blister packs.”
Recall Details
This recall involves blister packages of prescription drugs from Novartis and Sandoz. The drugs are packaged with 3 to 10 tablets per blister card.
The recalled Novartis prescription blister packages have “Novartis,” the name of the drug, dosage, NDC, lot number and expiration date printed on the cartons and the blister cards. The recall includes the following:
| Recalled Novartis Prescription Drugs | NDC Numbers | Tablet Strength | Carton Configuration | Lot Numbers | Expiration Date |
|---|---|---|---|---|---|
| Zofran ODT® |
0078-0679-61 0078-0679-19 |
4 mg | 30 count: 3 cards with 10 tablets each |
1657088
|
Dec 2019
|
| Zofran ODT® |
0078-0680-61 0078-0680-19 |
8 mg | 30 count: 3 cards with 10 tablets each |
1641546
|
Oct 2019
|
| Entresto® (sacubitril/valsartan) |
0078-0659-61 0078-0659-35 |
24 mg/ 26 mg | 100 count: 10 cards with 10 tablets each |
FX000005 FX000004 FX000003 F0010 F0009 F0007 |
Apr 2020 Apr 2020 Sep 2019 Nov 2018 Aug 2018 Jul 2018 |
| Entresto® (sacubitril/valsartan) |
0078-0777-61 0078-0777-35 |
49 mg/ 51 mg | 100 count: 10 cards with 10 tablets each |
FX000001 F0006 F0005 F0004 |
Dec 2019 Oct 2019 Aug 2019 Oct 2018 |
| Entresto® (sacubitril/valsartan) |
0078-0696-61 0078-0696-35 |
97 mg/ 103 mg | 100 count: 10 cards with 10 tablets each |
FX000002 F0007 F0006 F0005 F0004 |
Mar 2020 Feb 2020 Dec 2019 Dec 2018 Oct 2018 |
The recalled Sandoz prescription blister packages have “Sandoz,” the name of the drug, dosage, NDC and lot number printed on the cartons and the blister cards.
Lot numbers are listed at www.us.sandoz.com/patients-customers/product-safety-notices.
The recall includes the following:
| Recalled Sandoz Prescription Drugs | Tablet Strength | NDC Numbers | Carton Configuration |
|---|---|---|---|
| Azithromycin Tablets | 250 mg |
0781-5776-06 0781-5776-69 |
50 count: 5 cards with 10 tablets each |
| Donepezil ODT Tablets | 5 mg |
0781-5276-06 0781-5276-64 |
30 count: 3 cards with 10 tablets each |
| Donepezil ODT Tablets | 10 mg |
0781-5277-06 0781-5277-64 |
30 count: 3 cards with 10 tablets each |
| Haloperidol Tablets | 0.5 mg | 0781-1391-13 | 100 count: 10 cards with 10 tablets each |
| Haloperidol Tablets | 1 mg | 0781-1392-13 | 100 count: 10 cards with 10 tablets each |
| Haloperidol Tablets | 2 mg | 0781-1393-13 | 100 count: 10 cards with 10 tablets each |
| Haloperidol Tablets | 5 mg | 0781-1396-13 | 100 count: 10 cards with 10 tablets each |
| Haloperidol Tablets | 10 mg | 0781-1397-13 | 100 count: 10 cards with 10 tablets each |
| Imipramine HCl Tablets | 25 mg | 0781-1764-13 | 100 count: 10 cards with 10 tablets each |
| Imipramine HCl Tablets | 50 mg | 0781-1766-13 | 100 count: 10 cards with 10 tablets each |
| Isosorbide Dinitrate (ISDN) Tablets | 10 mg | 0781-1556-13 | 100 count: 10 cards with 10 tablets each |
| Isosorbide Dinitrate (ISDN) Tablets | 20 mg | 0781-1695-13 | 100 count: 10 cards with 10 tablets each |
| Naratriptan Tablets | 2.5 mg |
0781-5527-06 0781-5527-37 |
9 count: 1 card with 9 tablets |
| Ondansetron Tablets | 8 mg | 0781-1681-33 | 3 count: 1 card with 3 tablets |
| Ondansetron ODT | 4 mg |
0781-5238-06 0781-5238-64 |
30 count: 3 cards with 10 tablets each |
| Ondansetron ODT | 8 mg |
0781-5239-06 0781-5239-64 |
30 count: 3 cards with 10 tablets each |
| Ondansetron ODT | 8 mg |
0781-5239-06 0781-5239-80 |
10 count: 1 card with 10 tablets |
| Perphenazine Tablets | 2 mg | 0781-1046-13 | 100 count: 10 cards with 10 tablets each |
| Perphenazine Tablets | 4 mg | 0781-1047-13 | 100 count: 10 cards with 10 tablets each |
| Perphenazine Tablets | 8 mg | 0781-1048-13 | 100 count: 10 cards with 10 tablets each |
| Risperidone ODT | 0.5 mg |
0781-5310-06 0781-5310-08 |
28 count: 7 cards with 4 tablets each |
| Risperidone ODT | 1 mg |
0781-5311-06 0781-5311-08 |
28 count: 7 cards with 4 tablets each |
| Risperidone ODT | 2 mg |
0781-5312-06 0781-5312-08 |
28 count: 7 cards with 4 tablets each |
| Risperidone ODT | 3 mg |
0781-5313-06 0781-5313-08 |
28 count: 7 cards with 4 tablets each |
| Risperidone ODT | 4 mg |
0781-5314-06 0781-5314-08 |
28 count: 7 cards with 4 tablets each |
Consumers should immediately secure the blister cards to keep them out of the sight and reach of children and contact Novartis or Sandoz for further instructions. Novartis and Sandoz advise that consumers should continue to use the medication as directed once the blister packages are secured.
The firms have received one report of a child ingesting haloperidol from a blister pack.
Note: Individual Commissioners may have statements related to this topic. Please visit www.cpsc.gov/commissioners to search for statements related to this or other topics.
If you are experiencing issues with a recall remedy or believe a company is being non-responsive to your remedy request, please use this form and explain the situation to CPSC.
The U.S. Consumer Product Safety Commission (CPSC) is charged with protecting the public from unreasonable risk of injury associated with the use of thousands of types of consumer products. Deaths, injuries, and property damage from consumer product-related incidents cost the nation more than $1 trillion annually. Since the CPSC was established more than 50 years ago, it has worked to ensure the safety of consumer products, which has contributed to a decline in injuries associated with these products.
Federal law prohibits any person from selling products subject to a Commission ordered recall or a voluntary recall undertaken in consultation with the CPSC.
For lifesaving information:
- Visit CPSC.gov.
- Sign up to receive our email alerts.
- Follow us on Facebook, Instagram, X, BlueSky, Threads, LinkedIn and Truth Social.
- Report a dangerous product or product-related injury on www.SaferProducts.gov.
- Call CPSC’s Hotline at 800-638-2772 (TTY 800-638-8270).
- Contact a media specialist.