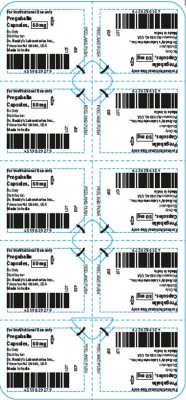
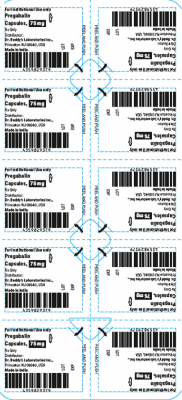
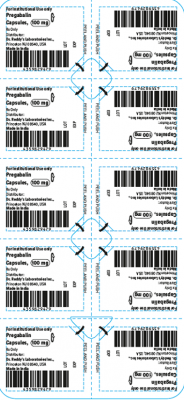
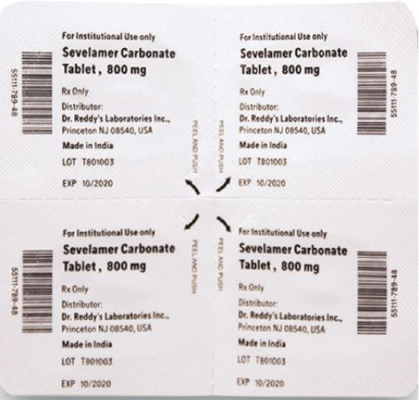
The products are prescription medications that were labeled and distributed by Dr. Reddy’s for institutional use only. The prescription medications were distributed by third party wholesalers to retail pharmacies and could have been dispensed to consumers. The packaging of the products is not child resistant and can pose a risk of poisoning if the contents are swallowed by young children.
About 21,400
Dr. Reddy’s toll-free at 888-375-3784 from 8 a.m. to 8 p.m. ET Monday through Friday, or online at www.drreddys.com and click on “Recall” for more information. Report incidents related to children accessing or ingesting these prescription medications to www.SaferProducts.gov. Report adverse events, medication errors, and quality problems related to the use of these products to FDA’s MedWatch Adverse Event Reporting Program either online at www.fda.gov/medwatch/report.htm, download the form at www.fda.gov/MedWatch/getforms.htm or call 800-332-1088 to request a reporting form, then complete and submit by regular mail, and return to the address on the pre-addressed form, or submit by fax to 800-FDA-0178.
Recall Details
This recall involves blister packages of prescription medications. The name and strength of the medication, “For Institutional Use only,” “Rx Only,” lot number and expiration date are printed on the outside of the package as well as on the individual blister units. The Dr. Reddy’s logo and NDC number are printed on the outside of the package. The recalled medications include the following:
|
Recalled Prescription Drugs
|
NDC Numbers |
Carton Configurations |
Lot Numbers |
Expiration Dates |
|
Imatinib Mesylate Tablets 100 mg |
43598-344-31
|
3 blister cards of 10 tablets
|
H2000138
|
2022-0630 |
|
Imatinib Mesylate Tablets 400 mg |
43598-345-31 |
3 blister cards of 10 tablets |
H2000127 |
2022-0630 |
|
Pregabalin Capsules 50 mg |
43598-292-66 |
5 blister cards of 10 capsules |
T900876 |
2021-0630 |
|
Pregabalin Capsules 75 mg |
43598-293-66 |
5 blister cards of 10 capsules |
T901021 |
2021-0731 |
|
Pregabalin Capsules 100 mg |
43598-294-66 |
5 blister cards of 10 capsules |
T901022 |
2021-0731 |
|
Pregabalin Capsules 150 mg |
43598-295-66 |
5 blister cards of 10 capsules |
T901023 |
2021-0731 |
|
Sevelamer Carbonate Tablets 800 mg |
55111-789-11 |
4 blister cards of 25 tablets |
T801003, T000009, T900221 |
2020-1031, 2021-1231, 2021-0228 |
|
Tadalafil Tablets 5 mg |
43598-575-31 |
3 blister cards of 10 tablets |
T000376 |
2022-0131 |
|
Tadalafil Tablets 20 mg |
43598-573-31 |
3 blister cards of 10 tablets |
T000425 |
2022-0228 |
Consumers should immediately store the recalled medications in a safe location out of reach of children and contact Dr. Reddy’s for a full refund.
No incidents or injuries have been reported.
Dr. Reddy’s Laboratories, Inc., of Princeton, N.J.
Note: Individual Commissioners may have statements related to this topic. Please visit www.cpsc.gov/commissioners to search for statements related to this or other topics.
If you are experiencing issues with a recall remedy or believe a company is being non-responsive to your remedy request, please use this form and explain the situation to CPSC.
The U.S. Consumer Product Safety Commission (CPSC) is charged with protecting the public from unreasonable risk of injury associated with the use of thousands of types of consumer products. Deaths, injuries, and property damage from consumer product-related incidents cost the nation more than $1 trillion annually. Since the CPSC was established more than 50 years ago, it has worked to ensure the safety of consumer products, which has contributed to a decline in injuries associated with these products.
Federal law prohibits any person from selling products subject to a Commission ordered recall or a voluntary recall undertaken in consultation with the CPSC.
For lifesaving information:
- Visit CPSC.gov.
- Sign up to receive our email alerts.
- Follow us on Facebook, Instagram, X, BlueSky, Threads, LinkedIn and Truth Social.
- Report a dangerous product or product-related injury on www.SaferProducts.gov.
- Call CPSC’s Hotline at 800-638-2772 (TTY 800-638-8270).
- Contact a media specialist.