Recall Details
| FOR IMMEDIATE RELEASE June 23, 2011 Release #11-258 |
Firm's Recall Hotline: (800) 258-2471 CPSC Recall Hotline: (800) 638-2772 CPSC Media Contact: (301) 504-7908 |
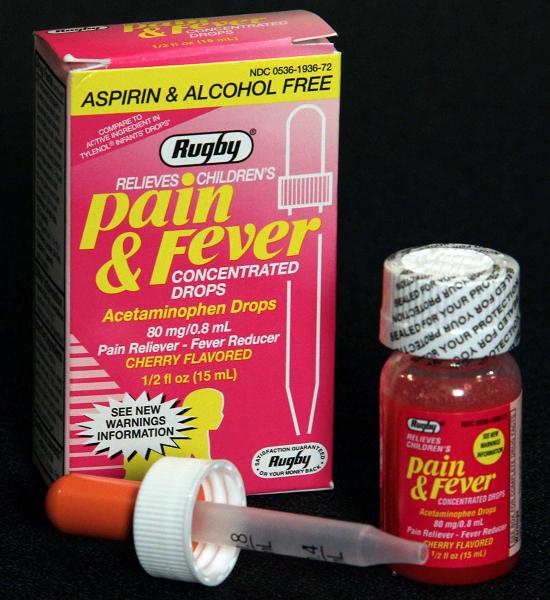
Rugby Children's Pain & Fever Concentrated Drops Recalled Due To Failure to Meet Child-Resistant Closure Requirement
WASHINGTON, D.C. - The U.S. Consumer Product Safety Commission, in cooperation with the firm named below, today announced a voluntary recall of the following consumer product. Consumers should stop using recalled products immediately unless otherwise instructed. It is illegal to resell or attempt to resell a recalled consumer product.
Name of Product: Children's Pain & Fever Concentrated Drops
Units: About 898,000
Manufacturer: Altaire Pharmaceuticals, Inc., of Aquebogue, N.Y
Distributed by: Rugby Laboratories, Inc., of Duluth, Georgia
Hazard: This over the counter medicine contains acetaminophen which calls for child-resistant packaging as required by the Poison Prevention Packaging Act. Although the original bottle has child-resistant packaging, a separate dropper unit provided for dispensing the drug to children does not. When in use, a child can access the medicine, posing serious health problems or death if more than the recommended dosage is consumed.
Incidents/Injuries: None reported
Description: The recall involves Rugby Children's Pain & Fever Concentrated Drops (Acetaminophen Drops) in a 1/2 fl. oz. (15 ml) bottle size. The UPC code 305361936723 can be found with the bar code at the bottom of the box. The affected lot numbers are:
| 09002 | 09379 | 10272 | 10368 | 10487 |
| 09131 | 09394 | 10273 | 10406 | 11058 |
| 09215 | 10154 | 10366 | 10433 |
The lot numbers can be found stamped into the bottom of the carton with the expiration date and above the label on the bottle printed in black.
Sold at: Drug stores, grocery stores and other retailers nationwide between January 2009 and June 2011 for about $4.
Manufactured in: United States
Remedy: Consumers should immediately store this product with the child-resistant closure in place and keep it out of the reach of children. To arrange for a free replacement dropper, contact Altaire Pharmaceuticals at (800) 258-2471 9 a.m. to 5 p.m. ET Monday through Friday.
Consumer Contact: For additional information, contact Rugby Laboratories at (800) 645-2158 between 9 a.m. and 5 p.m. ET Monday through Friday.
Note: Individual Commissioners may have statements related to this topic. Please visit www.cpsc.gov/commissioners to search for statements related to this or other topics.
The U.S. Consumer Product Safety Commission (CPSC) is charged with protecting the public from unreasonable risk of injury or death associated with the use of thousands of types of consumer products. Deaths, injuries, and property damage from consumer product-related incidents cost the nation more than $1 trillion annually. CPSC's work to ensure the safety of consumer products has contributed to a decline in the rate of injuries associated with consumer products over the past 50 years.
Federal law prohibits any person from selling products subject to a Commission ordered recall or a voluntary recall undertaken in consultation with the CPSC.
- Visit CPSC.gov.
- Sign up to receive our e-mail alerts.
- Follow us on Facebook, Instagram @USCPSC and Twitter @USCPSC.
- Report a dangerous product or product-related injury on www.SaferProducts.gov.
- Call CPSC’s Hotline at 800-638-2772 (TTY 301-595-7054).
- Contact a media specialist.








