When bent, these teething rings can fit into an infant's mouth and trigger a gagging reflex. This poses a risk of vomiting, which could result in choking and aspiration.
About 475,000
Consumers can call Whitehall-Robins Healthcare at (800) 525-2607 between 9 a.m. and 5 p.m. ET Monday through Friday.
Recall Details
WASHINGTON, D.C. - In cooperation with the U.S. Consumer Product Safety Commission (CPSC), Whitehall-Robins Healthcare, of Madison, N.J., is voluntarily recalling about 475,000 teething rings. When bent, these teething rings can fit into an infant's mouth and trigger a gagging reflex. This poses a risk of vomiting, which could result in choking and aspiration.
Whitehall-Robins Healthcare has received two reports from consumers of children gagging on the teething rings, including one report of a child starting to choke.
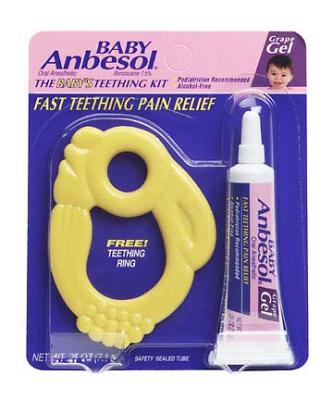
These teethers were sold with .25 oz. tubes of Baby Anbesol® Grape Gel, an oral anesthetic. The teethers are yellow and shaped like rabbits. They are about 3 inches long and 2.25 inches high. Two holes are molded into the teething ring for infants to grasp. The packaging reads, "THE BABY'S TEETHING KIT," and "FAST TEETHING PAIN RELIEF."
Mass merchandise, drug and grocery stores nationwide sold Baby Anbesol with these teethers from May 1999 through June 2000 for about $6.
Consumers should take these teething rings away from infants immediately. Contact Whitehall-Robins Healthcare for information on returning the teething ring in order to receive a free Anbesol® product. Consumers can call Whitehall-Robins Healthcare at (800) 525-2607 between 9 a.m. and 5 p.m. ET Monday through Friday, or go to the firm's website at http://www3.young-america.com/2506-2/Recall.asp.
Only the teething rings, and not the Baby Anbesol® Grape Gel, are being recalled.
Consumers should take these teething rings away from infants immediately. Contact Whitehall-Robins Healthcare for information on returning the teething ring in order to receive a free Anbesol® product.
Whitehall-Robins Healthcare has received two reports from consumers of children gagging on the teething rings, including one report of a child starting to choke.
Note: Individual Commissioners may have statements related to this topic. Please visit www.cpsc.gov/commissioners to search for statements related to this or other topics.
The U.S. Consumer Product Safety Commission (CPSC) is charged with protecting the public from unreasonable risk of injury or death associated with the use of thousands of types of consumer products. Deaths, injuries, and property damage from consumer product-related incidents cost the nation more than $1 trillion annually. CPSC's work to ensure the safety of consumer products has contributed to a decline in the rate of injuries associated with consumer products over the past 50 years.
Federal law prohibits any person from selling products subject to a Commission ordered recall or a voluntary recall undertaken in consultation with the CPSC.
- Visit CPSC.gov.
- Sign up to receive our e-mail alerts.
- Follow us on Facebook, Instagram @USCPSC and Twitter @USCPSC.
- Report a dangerous product or product-related injury on www.SaferProducts.gov.
- Call CPSC’s Hotline at 800-638-2772 (TTY 301-595-7054).
- Contact a media specialist.